
SL Paper 3
Ethylamine, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\), is a weak base.
State the equation for the reaction of ethylamine with water.
Explain why ethylamine has basic properties.
State the formula and deduce the shape of the positive ion (cation) formed when triethylamine, \({{\text{(}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{)}}_{\text{3}}}{\text{N}}\), reacts with hydrochloric acid.
Steel is an alloy of iron, carbon and other metallic and non-metallic elements. Stainless steel contains about 18% chromium and 8% nickel. Explain why iron can form alloys with other transition metals.
Lanthanum, La, and antimony, Sb, form compounds with bromine that have similar formulas, LaBr3 and SbBr3.
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
The table summarizes some properties of graphite and graphene.

Graphene is two-dimensional, rather than three-dimensional, material.
Justify this by using the structure of graphene and information from the table.
Show that graphene is over 1600 times stronger than graphite.
Identify a value from the table which can be used to support the information about graphene given below.
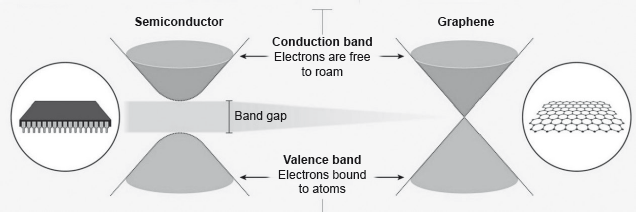
Electrons in a solid are restricted to certain ranges, or bands, of energy (vertical axis). In an insulator or semiconductor, an electron bound to an atom can break free only if it gets enough energy from heat or a passing photon to jump the “band gap”, but in graphene the gap is infinitely small.

Diamond, graphene, and graphite are all network solids.
Suggest, giving a reason, the electron mobility of diamond compared to graphene.
The melting point of diamond at 1 × 106 kPa is 4200 K (in the absence of oxygen).
Suggest, based on molecular structure, why graphene has a higher melting point under these conditions.
Sunflower oil contains stearic, oleic and linoleic fatty acids. The structural formulas of these acids are given in section 34 of the data booklet.
Explain which one of these fatty acids has the highest boiling point.
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 mol\(\,\)dm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
Many lipids are found in the human body. One type of lipid is a triglyceride.
Steroids and phospholipids are both classes of lipid found in the body. Cholesterol is a steroid. A structure of lecithin, a phospholipid, is shown below.

The formulas of some fatty acids are shown in Table 22 of the Data Booklet. State the equation for the reaction between glycerol and stearic acid to form a triglyceride.
Compare the structures of the two fatty acids: linoleic and linolenic acids.
State why these two fatty acids are so important in the human diet.
Distinguish between HDL and LDL cholesterol.
Compare the composition of cholesterol with a phospholipid such as lecithin.
Determine whether cholesterol or lecithin is more soluble in water.
Aluminium and iron are both widely used in modern society.
Almost all iron is used in the form of an alloy. State the name of the most common type of iron alloy and the other element that is an essential component of these alloys.
Name:
Other element:
An early alloy of aluminium was Duralumin which contained small quantities of copper and magnesium. This is stronger and more rigid than pure aluminium. Explain on an atomic level why the addition of other elements has this effect.
Linoleic acid is an essential fatty acid whose formula is given in Table 22 of the Data Booklet. Determine the mass of iodine, I2, which reacts with 100 g of linoleic acid.
Fats, such as butter, are solid triglycerides. Explain why fats have a higher energy value than carbohydrates.
The formula of stearic acid is also given in Table 22 of the Data Booklet. Explain why linoleic acid has a lower melting point compared to stearic acid.
Materials science involves understanding the properties of materials and applying those properties to desired structures.
Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name of the predominant type of bonding in each material.
Predict the predominant type of bonding for a binary compound AB in which the electronegativity of both atoms is low. Use section 29 of the data booklet.
The formula of linoleic acid is given in Table 22 of the Data Booklet.
Identify the structural formula of the triglyceride formed when three molecules of linoleic acid react with one molecule of glycerol (propane-1,2,3-triol), \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
State the other product formed during this reaction.
Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a solid at room temperature.
Describe how the triglyceride formed from linoleic acid and glycerol could be converted into a saturated fat and give any necessary conditions.
Other than the fact that it is a solid at room temperature, discuss two advantages and two disadvantages of a saturated fat compared to an unsaturated fat or oil.
Advantages:
Disadvantages:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
The structures of morphine, diamorphine and codeine are given in section 37 of the data booklet.
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
Suggest a reagent used to prepare diamorphine from morphine.
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
Vitamins can be water-soluble or fat-soluble.
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
State one function of vitamin D in the body.
The mild analgesic aspirin can be prepared in the laboratory from salicylic acid.
(CH3CO)2O + HOC6H4COOH → CH3CO2C6H4COOH + CH3COOH
Salicylic acid Aspirin
After the reaction is complete, the product is isolated, recrystallized, tested for purity and the experimental yield is measured. A student’s results in a single trial are as follows.
Literature melting point data: aspirin = 138–140 °C
Determine the percentage experimental yield of the product after recrystallization. The molar masses are as follows: M(salicylic acid) = 138.13 g mol−1, M(aspirin) = 180.17 g mol−1. (You do not need to process the uncertainties in the calculation.)
Suggest why isolation of the crude product involved the addition of ice-cold water.
Justify the conclusion that recrystallization increased the purity of the product, by reference to two differences between the melting point data of the crude and recrystallized products.
State why aspirin is described as a mild analgesic with reference to its site of action.
Polymers are made up of repeating monomer units which can be manipulated in various ways to give structures with desired properties.
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
Deduce the percentage atom economy for polymerization of 2-methylpropene.
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
Peptidase enzyme in the digestive system hydrolyses peptide bonds.
A tripeptide Ala-Asp-Lys was hydrolysed and electrophoresis of the mixture of the amino acids was carried out at a pH of 6.0. Refer to section 33 of the data booklet.
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
Identify the name of the amino acid that does not move under the influence of the applied voltage.
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
Outline how lead ions could be removed from an individual suffering from lead poisoning.
Lipids and carbohydrates contain the same elements but have different properties.
List the building blocks of triglycerides and carbohydrates.
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16.
Lactose is a disaccharide formed by the condensation reaction of the monosaccharides galactose and glucose.
Describe what is meant by a condensation reaction.
Draw the structure of galactose on the skeleton provided.
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
Solubility plays an important role in the bioavailability of drugs in the body.
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
Lipids are an important part of the human diet.
Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed in this reaction and the name of the other product.
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
The development of materials with unique properties is critical to advances in industry.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
Infrared (IR) spectroscopy is often used for the identification of polymers, such as PETE, for recycling.
LDPE and high density polyethene (HDPE) have very similar IR spectra even though they have rather different structures and physical properties.
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
Describe the difference in their structures.
Explain why the difference in their structures affects their melting points.
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.

When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.

Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.